Paris Services
Paris Control Systems offers a full quality assurance service for the development and testing of automation systems within the pharmaceutical industry
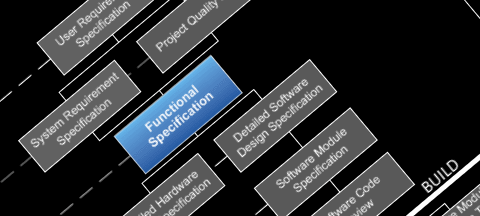
Our Services
Validation & Testing Services
The pharmaceutical industry has stringent validation and quality assurance requirements to which all control system software must comply before it can be deployed on active plant. Paris Control Systems has extensive experience and expertise in validating control systems for the pharmaceutical industry.
All our engineering and software development for the pharmaceutical industry is compliant with the QA requirements dictated by the Medicines and Healthcare products Regulatory Agency (MHRA) and, where appropriate, the American Food and Drug Administration (FDA). We adopt the standards and recommended practices required by the pharmaceutical industry as follows:
- GAMP 5 Good Automated Manufacturing Practice
- ANSI/ISA-88 standards for Batch Control
- ANSI/ISA-95 standards for Control System Integration
- ANSI/ISA-99 standards for Control System Security
All pharmaceutical projects implemented by Paris Control Systems follow the standard Life Cycle Model for design, build and verification required under the Good Automated Manufacturing Practice guide lines:
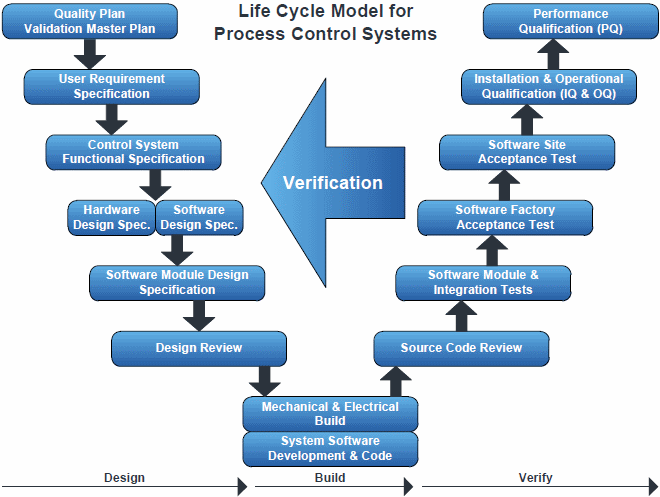
Paris Control Systems has its own quality procedures and is able to undertake all aspects of the Life Cycle Model. We are also able to provide independent testing and validation of third party control systems (either new build, or existing plant).
